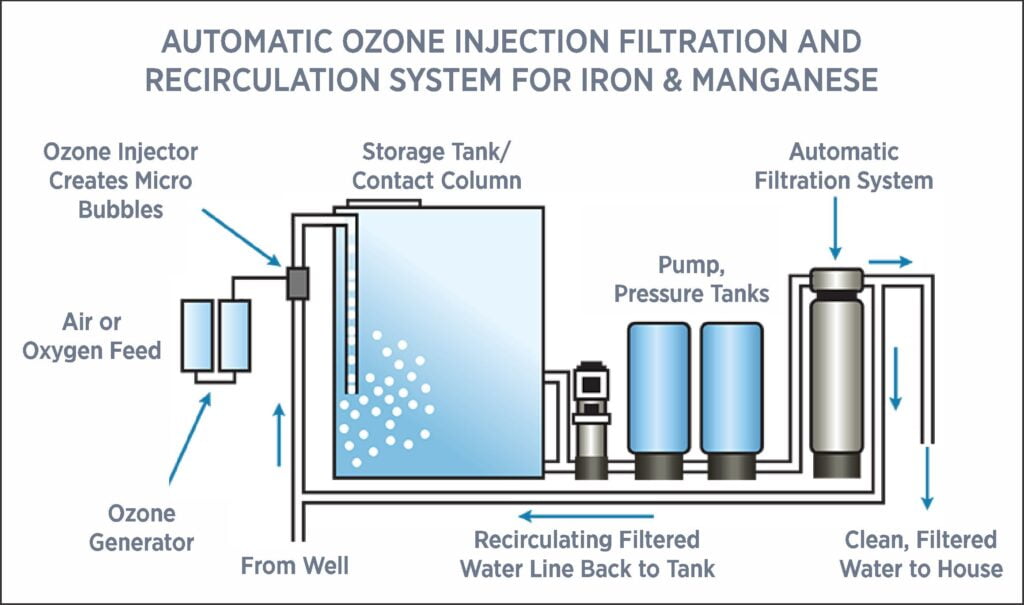
Ozone Solutions
Pacific Water Technology supplies a range of ozone generators for dissolution in water or gaseous applications for various sectors of industry. This also includes portable sanitation systems and also monitoring systems for the detection of ozone in the atmosphere and also dissolved ozone in aqueous solutions. For larger systems, we supply complete packages including oxygen concentrators to increase the efficiency of ozone generation. Below follows a list of applications in the Food Industry.
How Ozone Disinfects?
Ozone functions as both an oxidant and disinfectant in the treatment of drinking (potable) water and wastewater. This is similar to chlorine. Chlorine and Ozone, however, operate by different mechanisms when disinfecting water. As a result, ozone and chlorine can act synergistically.
Ozone’s germicidal properties are associated with its high oxidation potential. Disinfection by ozone is a direct result of bacterial cell wall disintegration, also known as lysis. This mechanism is different than that of chlorine. Although the exact chemical action of chlorine is not clear, it is believed that the chlorine residual in an aqueous solution diffuses through the cell wall of the microorganisms and attacks the enzyme group which results in the destruction of the microorganism.
Food and Beverage Industry Applications:
- Ozone use in CIP
- Ozone for surface sanitation
- Ozone for seafood processing
- Ozonated ice
- Ozone in fruit and vegetable processing
- Listeria inactivation with ozone
- reduction in ozone
- Food storage with ozone
- Portable sanitation systems for wine barrels, tanks and other POU sanitation applications.
The portable ozone cart systems incorporate high-concentration ozone/oxygen generation systems with an onboard pump, compressor, and mass transfer system in a rugged, all-stainless steel package. Simply connect plant water in and get a high concentration of ozonated water out with no loss of flow or pressure.
- Bottling filling plants and ozone sanitation
OZONE IN COOLING WATER
The key to an effective cooling water treatment programme includes management of the interrelationship between corrosion, scale/deposit and biological activity (micro and macro). For this reason treatment programmes normally include Orthophosphates, polymers and free halogens. Ozone can complement an existing water treatment programme by reducing chemical costs and inventory and reacts synergistically with other halogen-based oxidisers.
Ozone Advantages
- A factor of 10 to 100 times more germicidal than chlorine
- Reduces the use of chemicals
- May reduce discharge liabilities
- Destroys all types of microorganisms instantly
- Less risk of microbes building resistance to ozone treatment.
- Minimises condenser fouling
- Decomposes organic waste by oxidation
- Removes existing calcium carbonate scale by destroying the biomass glue bonding agent
- Low maintenance costs
- The most environmentally friendly oxidant
- No persistent chemicals or disinfectants in bleed – ozone breaks down to oxygen
- Reduces the corrosion rate of metals, including copper heat exchangers
- Saves on energy costs by increasing the heat transfer efficiency of the chiller
Ozone Reduces Costs in the Following Ways:
- Reducing makeup water to the cooling tower by permitting more cycles between blowdowns
- Reducing the cost of regular chemicals and fewer issues with wastewater disposal.
- Reducing power consumption by keeping the chiller heat transfer efficiency high through cleaner condenser tubes
OZONE IN GROUND WATER REMEDIATION
Chemical oxidation uses chemicals called “oxidants” to help change harmful contaminants into less toxic ones. It is commonly described as “in situ” because it is conducted in place, without having to excavate soil or pump out groundwater for above-ground clean-up.
In situ chemical oxidation, or “ISCO,” can be used to treat many types of contaminants like fuels, solvents and pesticides. ISCO is usually used to treat soil and groundwater contamination in the source area where contaminants were originally released. The source area may contain contaminants that have not yet dissolved into groundwater. Following ISCO, other clean-up methods, such as pump and treat or monitored natural attenuation, are often used to clean up the smaller amounts of contaminants left behind. Several oxidising agents can be used including ozone and hydrogen peroxide (or a mixture of both, or in the presence of a catalyst).
Ozone Advantages
- Twelve times more soluble than oxygen in the water
- Moves easily through the soil
- Produced on-site, with no need for hazardous chemical transportation or storage
- Most powerful oxidiser available
- Complex organics break down to carbon dioxide or less toxic molecules
- Breaks down to oxygen which increases DO levels
Contaminants Destroyed by Ozone
- MTBE (methyl tertiary butyl ether)
- BTEX (benzene, toluene, ethyl benzene, & xylenes)
- Hydrocarbons
- Diesel Fuel
- TCE (trichloroethylene)
- Pesticides
- Chlorinated Solvents
- VOCs (volatile organic compounds)
- Aliphatic & Poly-aromatic Hydrocarbons
OZONE & AQUACULTURE
Ozone is increasingly used in aquaculture due to its numerous advantages over traditional water treatment methods. Supplementing or replacing an existing system, ozone implementation has the potential to boost the competitive advantage of your aquaculture application. Ozone oxidation can kill microorganisms but requires maintaining a certain dissolved ozone concentration in the water for a given contact time. Disinfecting efficiency depends upon the product of the ozone residual concentration and its contact time. An ozone contact vessel provides the time necessary for the ozone residual to react with and inactivate pathogenic microorganisms.
Disinfecting waters may require maintaining a residual ozone concentration of 0.1–2.0 mg/L in a plug-flow type contact vessel for periods of 1–30 min, depending upon the target microorganism (Wedemeyer,1996).
Ozone Advantages
- Reduced Water Usage
- Faster Growth Rates
- Reduction of Waterborne Diseases
- Higher Standard of Environmental Control
- Supplements for other Treatment Processes
Why Ozone Use is growing in Aquaculture?
- It effectively removes organics, pesticides, discolouration, and nitrates.
- Typically, unconsumed ozone reverts back to oxygen, leaving no harmful residuals behind.
- Ozone oxidizes long-chain molecules, which are unaffected by biofiltration.
- Ozone involves a far lower risk of accidental pollution in comparison to other water treatment methods.
- O3 improves the effectiveness of biological and particulate filtration.
OZONE DISINFECTION
Ozone Disinfection/Ozone Contact Time Kinetics
Water is disinfected but never completely sterilized in the water treatment process. This disinfection is a two-part process that includes:
Courtesy of Eric Karch and David Loftis
- Removal of particulate matter by filtration. A rule of thumb is that high turbidity in the effluent is a potential health risk because viruses and bacteria can hide within the rough texture of particulates. Therefore, the removal of the particulates reduces the chance of pathogenic microorganisms in the effluent. (Refer to Figure 1)
- Inactivation of pathogenic microorganisms by chlorine, chlorine dioxide, ozone, or other disinfectants: Contact time and kinetics are simply a measure of the inactivation due to time and concentration of the disinfectant. The USEPA has developed regulations for the minimum kill percentages (inactivation) necessary for public water to be considered potable. These regulations include a minimum disinfection of:
- Three log (99.9%) for Giardia Lamblia cysts
- Four log (99.99%) for enteric viruses
In “water treatment terms” 1 log inactivation is referred to as 1 credit inactivation. Different types of filtration are assigned certain removal credits. For example, conventional filtration is worth 2.5 credits for Giardia cysts. Since the EPA requires 3 log (credit) removal, an additional 0.5 credit inactivation from disinfection must be attained.
[Figure 1: Simplified Diagram of a Pathogen Encapsulated by a Particulate]
Varying degrees of disinfection can be attained by altering the type and concentration of disinfectant, as well as the time water is in contact with the disinfectant. The decision to use one type of disinfectant versus another will set the precedence for the remainder of the values needed to attain the proper disinfection. The time untreated water is exposed to the disinfectant and the concentration of that disinfectant are the main factors in the equation that will be discussed in the next section. [Notice that the units of contact time are (mg/l)(min).]
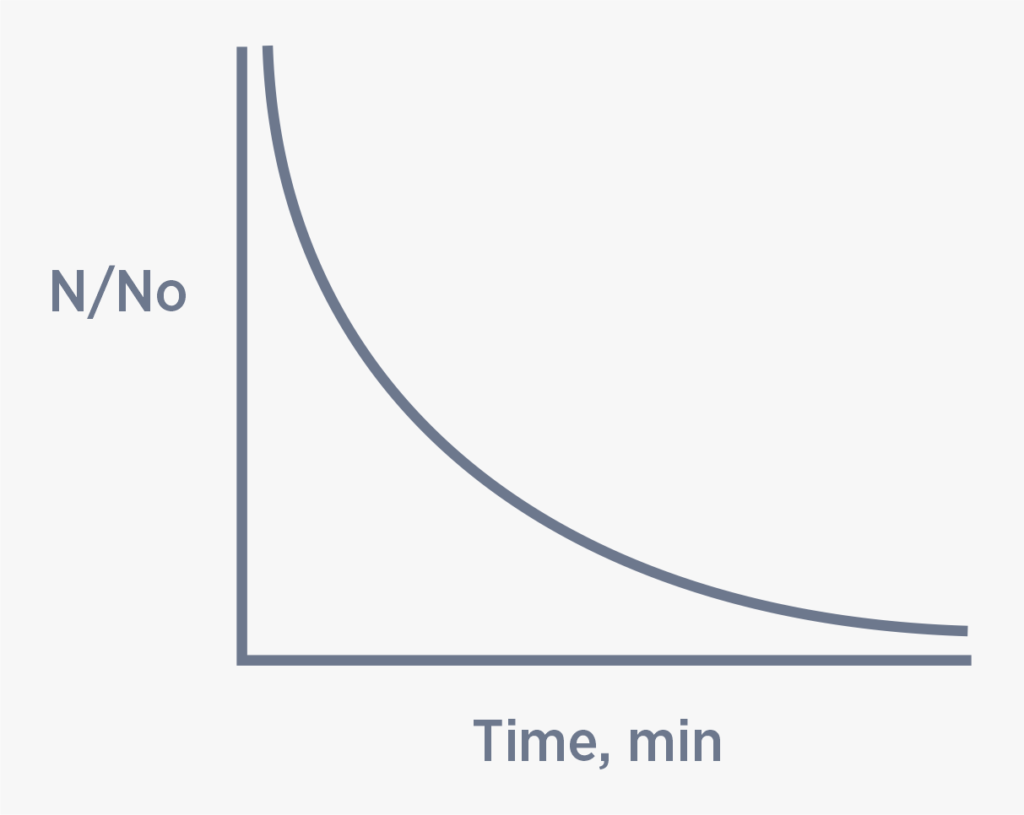
RELATIONSHIP BETWEEN KILL EFFICIENCY & CONTACT TIME
A relationship between kill efficiency and contact time was developed by Harriet Chick while she was a Fellow at the Pasteur Institute in Paris, France. The research yielded data supporting her relationship which is shown in Figure 2. (No) represents the initial number of organisms and N is the number of organisms at time t. As contact time between water and disinfectant increases, the ratio of No/N decreases as Chick’s Law predicts.
Factors Affecting C*t Values
- As pH increases the value of C*t also needs to be increased. This can be explained by examining the effects of pH on free chlorine. As the pH increases, more of the weak disinfectant (OCl-) exists than the strong disinfectant (HOCl-), thus increasing the C*t value. Refer to Table 1 below.
- The greater log removal needed, the greater the C*t needs to be, as can be seen in Table 1.
Table 1: C*t for Removal of Giardia Cysts in Relation to Log Removal and pH