Description
About Water Softening Resins
Ion exchangers are resins that are polymers with cross-linking ( connections between long carbon chains in a polymer ). The resin has active groups in the form of electrically charged sites. At these sites, ions of opposite charge are attracted but may be replaced by other ions depending on their relative concentrations and affinities for the sites. Two key factors determine the effectiveness of a given ion exchange resin: favourability of any given ion, and the number of active sites available for this exchange. To maximize the active sites, significant surface areas are generally desirable. The active sites are one of a few types of functional groups that can exchange ions with either plus or minus charge. Frequently, the resins are cast in the form of porous beads.
Cross-linking, usually on the order of 0.5 to 15 percent, comes from adding divinyl benzene to the reaction mixture during production of the resin. The size of the particles also plays a role in the utility of the resin. Smaller particles usually are more effective because of increased surface area but cause large head losses that drive up pump equipment and energy costs. Temperature and pH also affect the effectiveness of ion exchange, since pH is inherently tied to the number of ions available for exchange, and temperature governs the kinetics of the process. The rate-limiting step is not always the same, and temperature’s role is still not thoroughly understood.
Regeneration of the resin is also a feauture of ion exchange. The resin is flushed free of the newly-exchanged ions and contacted with a solution of the ions to replace them. Regeneration is initiated after most of the active sites have been used and the ion exchange is no longer effective. With regeneration, the same resin beads can be used over and over again, and the ions that we are looking to get out of the system can be concentrated in the backwash effluent, which is just a term for the spent fluid used to regenerate the ion exchanger.
Nowadays, the ion exchange substances are used almost exclusively under the name of resins. There are two categories of resins: the resins of the gel type and those of the macroporous or loosely cross-linked type. Their basic structure is identical: the macromolecular structure is obtained in both cases by co-polymerization. The difference between them lies in their porosity.
| Gel type resins have a natural porosity limited to intermolecular distances. It is a microporous type structure |
|
Macroporous type resins have an additional artificial porosity which is obtained by adding a substance designed for this purpose. |
The exchanger is known as monofunctional if there is only one variety of radicals and it is called polyfunctional if the molecule contains various type of radicals.
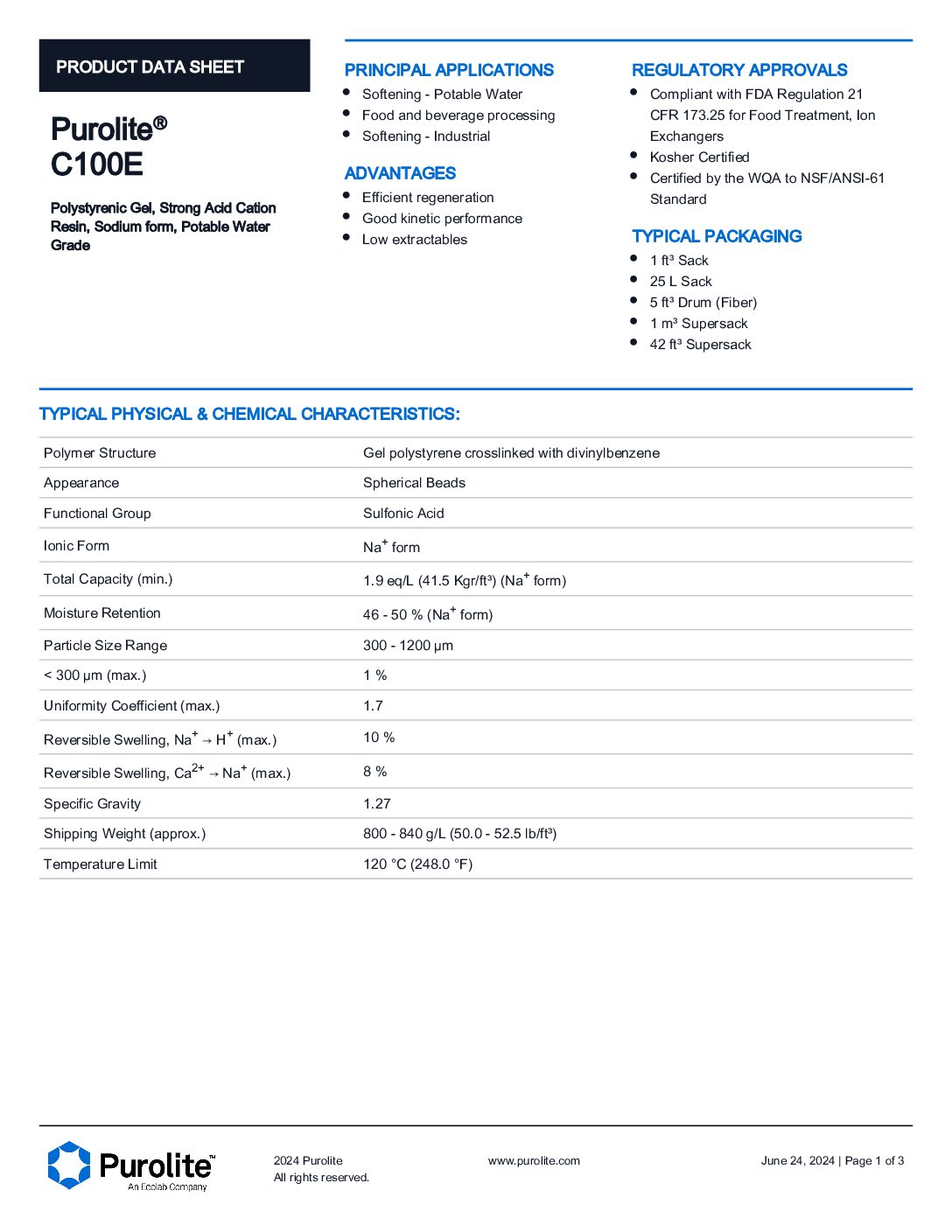
Purolite C100E Water Softening Resin
Main Applications
- Food and Beverage processing
- Softening potable water
- Used as a softener in industrial environment
Benefits
- Efficient regeneration
- Low extractables
- Good Kinetic performance
Purolite C100E Data sheet